The Ultimate Guide to Synthesizing Cetyl Trimethyl Ammonium Chloride: A Comprehensive Overview
Cetyl Trimethyl Ammonium Chloride (CTAC) is a vital chemical compound used in many industrial processes, such as pharmaceuticals, chemicals, and personal care. Understanding the synthesis process of this compound is crucial for professionals, chemists, and researchers in these industries. This article provides a comprehensive overview of synthesizing CTAC.
The Ultimate Guide to Synthesizing Cetyl Trimethyl Ammonium Chloride
To understand the synthesis of CTAC, this article provides detailed information on the materials required and the reaction steps for an efficient process.
1. Materials required for the synthesis of cetyl trimethyl ammonium chloride
Several critical materials are needed for the synthesis of CTAC. Some of these materials include the following:
- Cetyl alcohol: Cetyl alcohol is the primary starting material in the synthesis of CTAC. Also known as hexadecanol, cetyl alcohol can be obtained synthetically and from palm oil.
- Methyl chloride: Methyl chloride is a methylating agent that converts cetyl alcohol into triethyl ammonium chloride.
- Sodium hydroxide: Sodium hydroxide is another material required to synthesize CTAC. It is a strong base that helps formulate CTAC and facilitates the reaction.
- Hydrochloric acid: pH adjustment is required during the synthesis of CTAC. For this, hydrochloric acid is used.
- Solvent: A solvent is another essential material required for the synthesis of CTAC. A suitable solvent like water or an organic solvent like ethanol is needed for the reaction to occur.
2. Steps In Synthesizing Cetyl Trimethyl Ammonium Chloride
For efficient synthesis of CTAC, the proper procedure must be followed. Typically, below are the methods involved in the synthesis of CTAC:
Method 1: Methylation
One of the standard methods to synthesize CTAC is through methylation. The methylation reaction involves the conversion of cetyl alcohol into cetyl trimethyl ammonium chloride. The methylation reaction is typically carried out under specific conditions to ensure optimal conversion and yield. Catalyst, solvent, reaction time, temperature, and pressure are vital considerations for this reaction.
A catalyst that includes strong bases like Potassium Hydroxide or Sodium Hydroxide is employed to facilitate the reaction. A solvent is used to dissolve the reactants and create a reaction medium. Sufficient reaction time is necessary to allow for the completion of the methylation process. In contrast, the reaction is typically conducted at elevated temperatures and may require reflux or heating to ensure efficient conversion.
Method 2: Neutralization Reaction
This step involves the reaction of a cetyl amine precursor with hydrochloric acid. The formation of CTAC is the result of this reaction. CTAC and water are formed in this reaction when the cetyl amine precursor reacts with hydrochloric acid. The neutralization reaction for synthesizing CTAC requires specific conditions like reactant proportions, reaction temperature, and pH control to ensure efficient conversion and yield.
For the reactant proportion, the ratio of cetyl amine to hydrochloric acid is vital to achieve optimal conversion. Also, the neutralization reaction is typically carried out at ambient temperature or under controlled heating conditions. In contrast, the addition of hydrochloric acid is carefully controlled to achieve the desired pH for complete neutralization. pH monitoring is essential to ensuring efficient conversion.
The resulting mixture needs to go through workup and purification steps to isolate and purify the CTAC product. This process involves filtration and separation to separate the CTAC product from the reaction mixture, washing to remove residual impurities or reaction byproducts, and drying to remove any remaining moisture.
Method 3: Isolation
Another method for synthesizing CTAC involves the isolation of the compound from commercially available sources or natural materials. In the isolation process, the starting material for CTAC synthesis can be commercially available as CTAC or cetyl amine chloride. As another option, CTAC can be derived from natural sources such as coconut or palm oil by extracting the cetyl amine chloride precursor.
If the source material is natural, the extraction process involves isolating the cetyl amine chloride precursor from the oil or fat source. This can be achieved through various extraction methods, such as solvent extraction or steam distillation.
After the cetyl amine chloride precursor is obtained, it is further converted into CTAC through a chemical reaction. This typically involves the neutralization of cetyl amine chloride with a strong base, followed by the addition of hydrochloric acid to obtain CTAC and water.
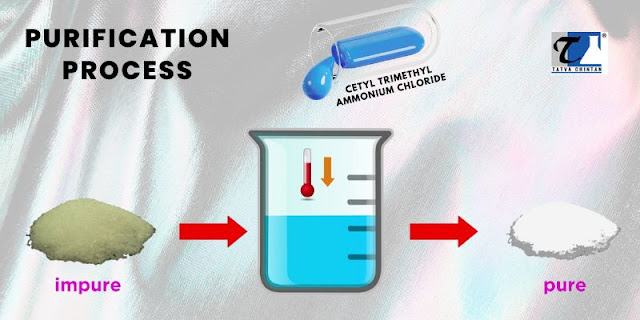
Method 4: Purification
Before initiating the purification process, the impure CTAC sample undergoes an analysis to identify and quantify the impurities present. This analysis helps determine the appropriate purification techniques and optimize the purification process.
Various purification techniques, such as recrystallization, chromatography, distillation, and solvent extraction, can refine the impure CTAC sample. The impure CTAC is dissolved in a suitable solvent and then allowed to crystallize. The crystals are separated, and the process is repeated to enhance purity.
Column chromatography, such as silica gel or ion exchange chromatography, can separate CTAC from impurities. If the impurities have different boiling points from CTAC, distillation techniques like fractional distillation or vacuum distillation can be used. The solvent extraction technique uses selective solvents to extract CTAC from impurities based on their differential solubilities.
Considerations and Safety When Synthesizing Cetyl Trimethyl Ammonium Chloride
Safety is key in the synthesis process. It is essential to consider the following when synthesizing CTAC:
- Handling Methyl Chloride and Sodium Hydroxide requires caution due to their hazardous nature. Proper personal protective equipment (PPE) should be worn, and the reaction should be conducted in a well-ventilated area.
- The reaction temperature, duration, and stoichiometry should be carefully controlled to ensure optimal conversion and yield.
- Regularly monitoring reaction progress and product quality through analytical techniques like titration, spectroscopy, or chromatography is essential to obtaining the desired product.
- Proper chemical waste disposal generated during synthesis should follow local regulations and guidelines to minimize environmental impact.
Conclusion
Synthesizing Cetyl Trimethyl Ammonium Chloride requires careful attention to the reaction steps, materials, and considerations outlined in this guide. By following the comprehensive overview provided, researchers and professionals can successfully produce CTAC, unlocking its wide range of applications across various industries.




Comments
Post a Comment